Multi-center acute myeloid leukemia study
An SWG Grant-supported project initiated by EHA's SWG on Acute Myeloid Leukemia.
Full project title
‘Evaluation of the prognostic impact of subclonal FLT3-ITD mutations in acute myeloid leukemia (AML): a retrospective multi-center study on behalf of the EHA AML-SWG.’
Project team
 Prof Maria Teresa Voso
Prof Maria Teresa Voso
Professor, Dipartimento di Biopatologia e Diagnostica per Immagini | Principal Investigator
University of Rome Tor Vergata
Italy
 Dr Carmelo Gurnari
Dr Carmelo Gurnari
M.D. | Co-Principal Investigator
Cleveland Clinic Cancer Center, Translational Hematology and Oncology Research
USA
Project background and aims
We plan to study the specific AML subset with subclonal FLT3-ITD mutations.
FLT3-ITD-mutated acute myeloid leukemia (AML) represents an unmet medical need. This is despite improvements in outcomes due to the introduction of FLT3-inhibitors in association with induction therapy.
FLT3-ITD mutations also represent a diagnostic challenge. Capillary electrophoresis has been confirmed by the most recent ELN guidelines as the method of choice to diagnose these AML subtypes.
The large RATIFY trial combined intensive chemotherapy with the FLT3 inhibitor midostaurin—so far, the only approved first-line FLT3 inhibitor in combination with chemotherapy. In this trial, the allelic ratio (AR) threshold to define FLT3-ITD positivity was conventionally set at 0.05. Therefore, FLT3-ITD mutational status has been based on this established cut-off.
However, recent data suggest that FLT3-ITD mutations:
- Maintain their negative prognostic potential
- Predict an increased risk of relapse, regardless of their baseline levels
Indeed, in over 60% of cases, patients with low-level positivity (AR<0.05) who are not candidates for FLT3-inhibitors present an FLT3-mutated relapse.
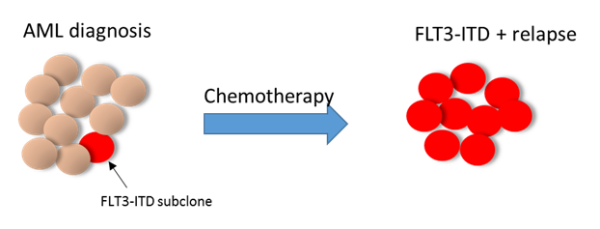
We plan to study the specific AML subset with subclonal FLT3-ITD mutations. This will involve a retrospective analysis of cases diagnosed at multiple European participating institutions, including several members of the EHA AML SWG.
Overall, the knowledge generated by this collaborative effort will:
- Shed light on the prognostic role of subclonal FLT3-ITD mutations
- Consolidate the thresholds for the definition of FLT3-ITD positivity at AML diagnosis
Our ultimate goal is to open the possibility that patients harboring FLT3-ITD subclones may be offered FLT3-inhibitors in association with standard treatment.
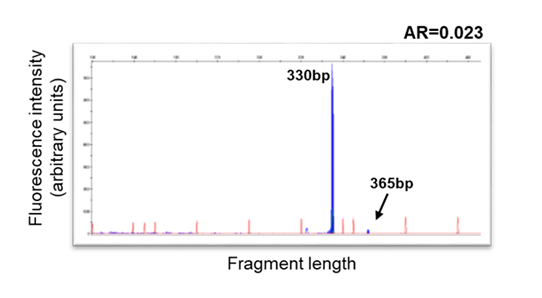
An example of sub-clonal FLT3-ITD at AML diagnosis.
Project updates
You can find out more about the status of this project by reading the latest update from Prof Maria Teresa Voso.



 Back
Back